Concentration of a solution refers to the weight or volume of solute in a specified amount of solution or solvent. According to chem.purdue.edu, it is a macroscopic property, meaning it describes the behaviors or characteristic of a sample which is large enough to see, weigh, manipulate, handle, etc.
It can be expressed in variety of methods:
1.) Specific Gravity is equal to:
since density is equal to mass/volume
Therefore Specific Gravity is equal to:
2.) Mass Percent refers to the mass of solute contributed to the total mass of solution. Mass Percent is equal to:
3.) Volume Percent refers to the volume of solute contributed to the total volume of solution. Volume Percent is equal to:
4.) Molarity (M) refers to the number of moles of solute present in a liter of solution. Molarity is equal to:
5.) Molality (m) refers to the number of moles of solute present in a kilogram of solvent. Molality is equal to:
6.) Normality (N) refers to the number of gram-equivalents of solute present in a liter of solution. Normality is equal to:
With the aid of the 6 formulas indicated above...
Have a try in solving the SAMPLE PROBLEMS:
2.) A pickling liquor is prepared by dissolving 215mL of concentrated HCl solution in 450mL of water. What percent of the liquor is acid?
3.) Calculate the normality of a solution prepared by dissolving 45.0g of barium hydroxide in enough water to make 500mL of solution.




































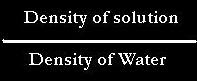







Sasarai | On: June 6, 2010 at 3:41 AM
Chemistry na naman! :)) Hahahahaha! ^_^ Babalikan ko ito. Muahahaha! *evil laugh*
Jhiegzh | On: June 6, 2010 at 3:44 AM
Bakit mo naman babalikan?
Nice Salcedo | On: June 6, 2010 at 4:26 AM
wow! haha..thanks so much for sharing! do we have to solve those problems? :D okay, i'll try! :) huhu..maybe i'll make a mistake.. :( correct me if i'm wrong! :D
1.) What is the molality of a solution prepared by dissolving 70.0g of glucose in 850g of water?
m = 70.0g/850g
m = 0.0824 g <<
2.) A pickling liquor is prepared by dissolving 215mL of concentrated HCl solution in 450mL of water. What percent of the liquor is acid?
215mL/450mL x 100% = 0.48 = 48% <<
3.) Calculate the normality of a solution prepared by dissolving 45.0g of barium hydroxide in enough water to make 500mL of solution.
N = 45.0g/500mL
N = 45.0g/5L
N = 9 g/L <<
Pordoy Palaboy | On: June 6, 2010 at 4:30 AM
nose hemorrhage...tabang nurse
Jhiegzh | On: June 6, 2010 at 4:37 AM
@Nice: Thanks for the try!...:) Uhmmm! Practice lang ^^
@Ghienox: Wooaaah!
Nice Salcedo | On: June 6, 2010 at 5:05 AM
@Jhiegzh: you're welcome! :D yeah! :) so, how are my answers? haha.. :)) are they correct?
Jag | On: June 6, 2010 at 5:25 AM
Shucks! 2.25 lng ako sa Chem1 at Chem2 hehehe...
Jhanz | On: June 6, 2010 at 5:29 AM
Wow. Chemistry. I never really learned a lot about chem back in high school. Glad I don't have to take it this college.
Ironically, my parents are both chemists. Haha. :)
Jhiegzh | On: June 6, 2010 at 5:58 AM
@NIce: Take note for your units! Unit for molality is (m) not g and for normality is N! BEfore you solve you need to determine first the number of moles!
@Jag: OK lang yan ah! Pasado naman yan dba?
@Umi: Wow, opposite pla kau ng parents mO! ^^
Nice Salcedo | On: June 6, 2010 at 6:40 AM
@Jhiegzh: ahh! okay! thanks! :) so i got correct on number 2? yay! haha.. :))
Jhiegzh | On: June 6, 2010 at 6:48 AM
@Nice: THis is a CHEM1 topic!
Martha Therese | On: June 6, 2010 at 6:48 AM
Waaa! Chemistry! i remember pinagalitan ako ng prof ko kasi di daw ako nakikinig! nagnonotes kasi ako tas dinadaldal pa ko ng seatmate ko. huhu. i hate chemistry. :(
Jhiegzh | On: June 6, 2010 at 8:16 AM
@BubbleS: Opposite here pero depends na rin sa topic! hahaha
Unknown | On: June 6, 2010 at 8:39 AM
Bagsak ako sa chemistry kaya di ako maka-relate .. haha.. tinutulugan ko lang yata to.. nosebleed ako sa formulas.. sa formula lang yata ng IVF computation ako hindi dinugo e.. whahaha
Jhiegzh | On: June 6, 2010 at 8:50 AM
@Mharliz: Aw ah! WOaaah!
Unknown | On: June 6, 2010 at 9:01 AM
haha.. e kasi naman.. kasi.. bobo talaga ako sa math.. ang alam ko lang me plus him equals love.. haha joke..
wala makulit ako ngaun.. wag ka ng umangal.. haha
Sasarai | On: June 6, 2010 at 11:15 AM
1] Given:
Mass of solute = 70g
Mass of solvent = 850g -> 0.85kg
Molecular Weight of C6H12O6 -> 180g/mol
mole of C6H12O6 = 70/180 = 0.389 moles
Molality = 0.389/0.85 = 0.458 molal
2] Given:
HCl = 215mL
H20 = 450mL
Applying Percent Volume formula, since we need the volume percentage of liquor (HCl), we have
450mL / 215mL * 100% = 2.09%
Even if you convert mL to L, the answer is still the same.
3] Given:
Ba(OH)2 = 45g
H20 = 500mL
Since we have a basic chemical (Barium Hydroxide), Normality can be solved by the formula
N = (Mass of solute/equivalent weight of base)/Liters of solution
to get the equivalent weight:
Formula weight / No. of available hydroxides per formula unit
171 / 2
= 86
substitute it to the normality formula:
(450/86)/500
we have: 0.0105N or equivalents/L.
I hope tama ako kasi poor ako sa Chemistry! T_T
Jhiegzh | On: June 6, 2010 at 8:56 PM
@Juls: Bakit mo kinuha ang molecular weight..Uhmm! I cant tell the answer! Pero nice try!
@Mharliz: Hindi tlga ako aangal! naks!
Superjaid | On: June 6, 2010 at 9:00 PM
ayokong sagutin to..hmp!
hehehe high school days ko pa to nakuha..i think 2nd year namin..kaya ayoko na sawa na ako sa chem..wahaha
Jhiegzh | On: June 6, 2010 at 9:04 PM
@Superjaid: Ok lang ah! I will not reveal the answer though!...^^
Keiyt | On: June 6, 2010 at 9:21 PM
Nakakainis. ;)))) I took Chem nung 1st sem last school year. Daaaaamnnn. Okay, nakalimutan ko agad. Haha. Pero hindi naman ako nagma-major niyan so okay lang. ;)) Nosebleed eh.
Jhiegzh | On: June 6, 2010 at 10:15 PM
@Keiyt: OK lang yan ah...Ako nga rn eh! LOL!
Nice Salcedo | On: June 6, 2010 at 11:36 PM
I love Chemistry! hahahaha.. :)) even though there are a lot of headache-giving formulas! waa..
Jhiegzh | On: June 7, 2010 at 12:47 AM
@Nice: We are same! ^^
Rose | On: June 7, 2010 at 2:09 AM
oh my..kuya jose naman hehehe i hate chem! prmise hehehhehe..5 units pa naman chem namin..gs2 ko lang pag lab..pero pag chem lec naku terror ang prof namin..yan din inaral namn last year..ngaun physics n kmi :)
Jhiegzh | On: June 7, 2010 at 3:16 AM
@Rose: Ok na nga yun eh! ^_^....:)
Sasarai | On: June 7, 2010 at 4:12 AM
Ewan ko nga ba eh. Hahahahaha! Baliw lang ako nung sinagutan yan! :PP
Jhiegzh | On: June 7, 2010 at 4:43 AM
@Juls: Uhmmm...Hahahaha
yvette | On: June 7, 2010 at 4:59 AM
skt sa ulo yang chem na yan grabe hehe :)
Jhiegzh | On: June 7, 2010 at 5:07 AM
@Yvette: Hehehe..Oo! Woaaah ^^
Biopolymath | On: June 10, 2010 at 11:47 PM
This certainly helps chemistry students! I've touched a lot on chemistry, the most common unit of concentration is the molarity. Instead of litre, I often express molarity in terms of cubic decimetre - but both are equivalent.
As I moved on to biochemistry, I encounter concerntrations in terms of micromolar and milimolar - to talk about DNA and protein samples!
Jhiegzh | On: June 11, 2010 at 1:05 AM
@Boon: Yeah! I knew that!